
Über uns

Ein Team – eine Mission
Unser Team führt und arbeitet mit Exzellenz und langjähriger Branchenerfahrung bei Weltmarktführern sowie Forschungseinrichtungen
Unser Medical Board bewertet und begleitet unsere Engineering-Projekte wie auch unsere klinischen Studien.
Das Ziel vor Augen
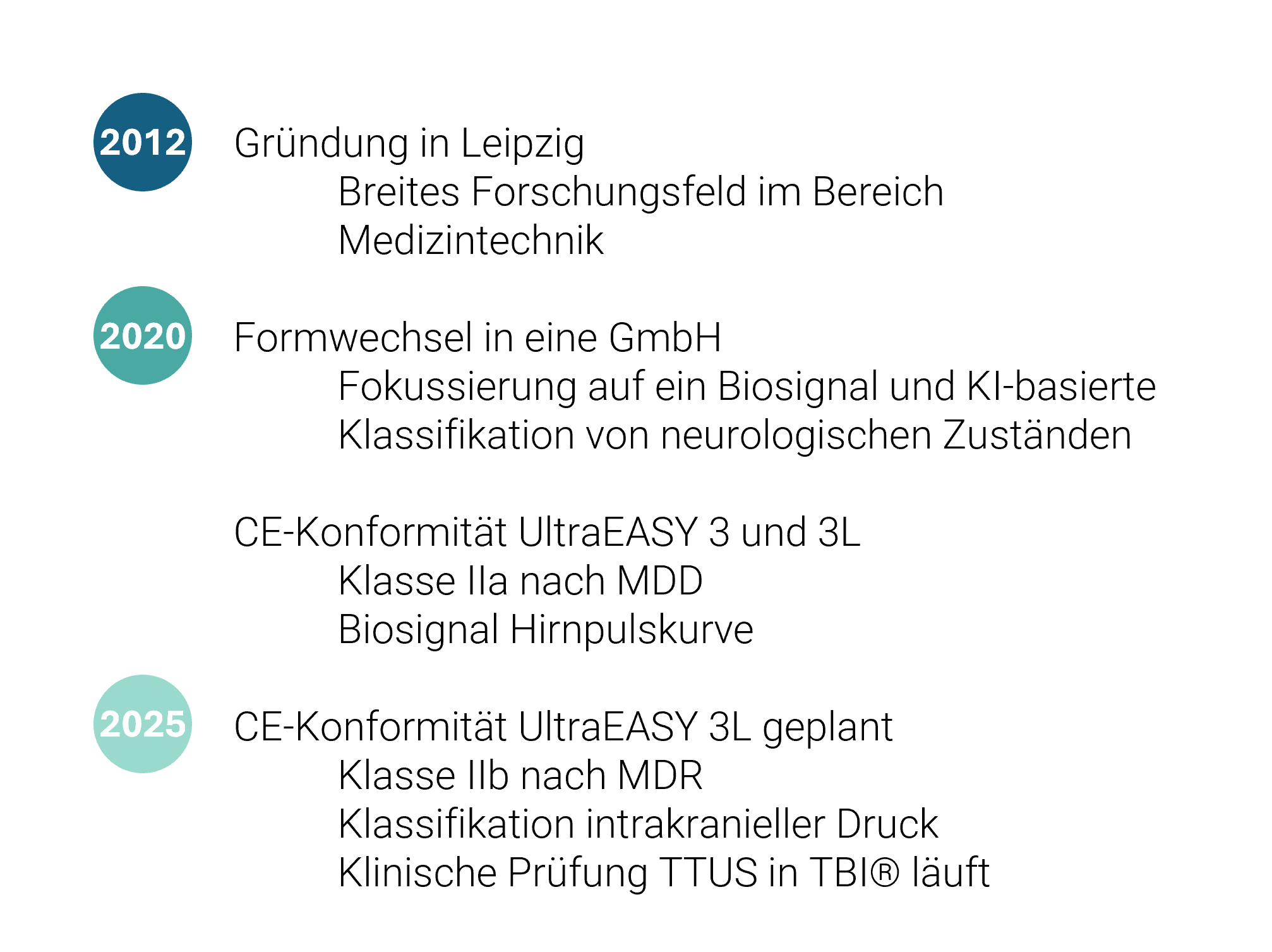
Der Nachfolger des UltraEASY 3L befindet sich in der klinischen Prüfung, TTUS in TBI®. Als erstes Medizinprodukt zur nicht-invasiven Klassifikation des intrakraniellen Drucks wird es voraussichtlich 2025 erhältlich sein.
*Transkranieller Transmissionsultraschall, TTUS®

Starke Partner
Sonovum nutzt ein Netzwerk aus exzellenten Partnern, um die Entwicklung innovativer Medizinprodukte voranzutreiben und Patienten bestmöglich zu versorgen.Dazu gehören Start Up und MedTech Plattformen, die den Austausch von Know-how und Erfahrungen fördern. Unsere Supplier liefern hochwertige und state-of-the-art Hardware-Komponenten für unsere Medizinprodukte, während wir durch Forschungskooperationen mit Fraunhofer-Instituten und technischen Universitäten anwendungsorientierte Lösungen entwickeln.
Zertifikate
Sonovum ist mit dem Qualitätsmanagementsystem nach ISO 13485 als Medizintechnikhersteller zertifiziert und unsere Medizinprodukte tragen das CE-Kennzeichen. Die erforderlichen Dokumente stehen hier zum Download bereit.